Polyolefins (Polyalkenes)
Properties
Polyolefins, also called polyalkenes, are the largest class of commodity thermoplastics. They are polymers of simple alkenes such as ethylene, propylene, butenes, and pentenes, and copolymers thereof. The two most important polyolefins are polyethylene (PE) and polypropylene (PP). They can be processed by common forming techniques such as injection molding, blow molding, extrusion and thermoforming, using standard thermoplastic equipment. Their easy processability and low price in combination with their good chemical and physical properties, makes them the most popular resins and often the best choice for a multitude of plastic applications.
Polyethylene (IUPAC name polymethylene) is the largest volume polyolefin. A variety of different grades are commercially available. These differ in molecular weight, crystallinity (density), and branching. Some special grades are also crosslinkable. The degree of branching as well as the length of the branches affects the density which can vary between 0.87 g/cm3 and 0.97 g/cm3. Typically, the higher the density of the polymer the higher the degree of crystallinity and the stiffer and harder the polymer.
Both the degree of branching and the polymer molecular weight and its distribution determine the mechanical properties and the melt flow behavior of polyethylene. High molecular weight polyethylene without branching is rather brittle. To increase the flexibility, ethlyene is typically copolymerized with low molecular weight alkenes such as butene-1, hexene-1 or octene-2 which introduces short chain branches on the mostly linear polymer chain. Many different grades are commercially available that differ in the degree of branching and molecular weight. The ten most common grades are:
- Ultra-high-molecular-weight polyethylene (HMWPE)
- High-molecular-weight polyethylene (HMWPE)
- High-density polyethylene (HDPE)
- High-density cross-linked (cross-linkable) polyethylene (HDXLPE)-
- Medium-density polyethylene (MDPE)
- Linear Medium-density polyethylene (LMDPE)
- Cross-linked (cross-linkable) polyethylene (PEX or XLPE)
- Low-density polyethylene (LDPE)
- Linear low-density polyethylene (LLDPE)
- Very-low-density polyethylene (VLDPE)
- Ultra-low-molecular-weight polyethylene (PE-WAX)
Among these, HDPE, LLDPE and LDPE are the highest volume grades.
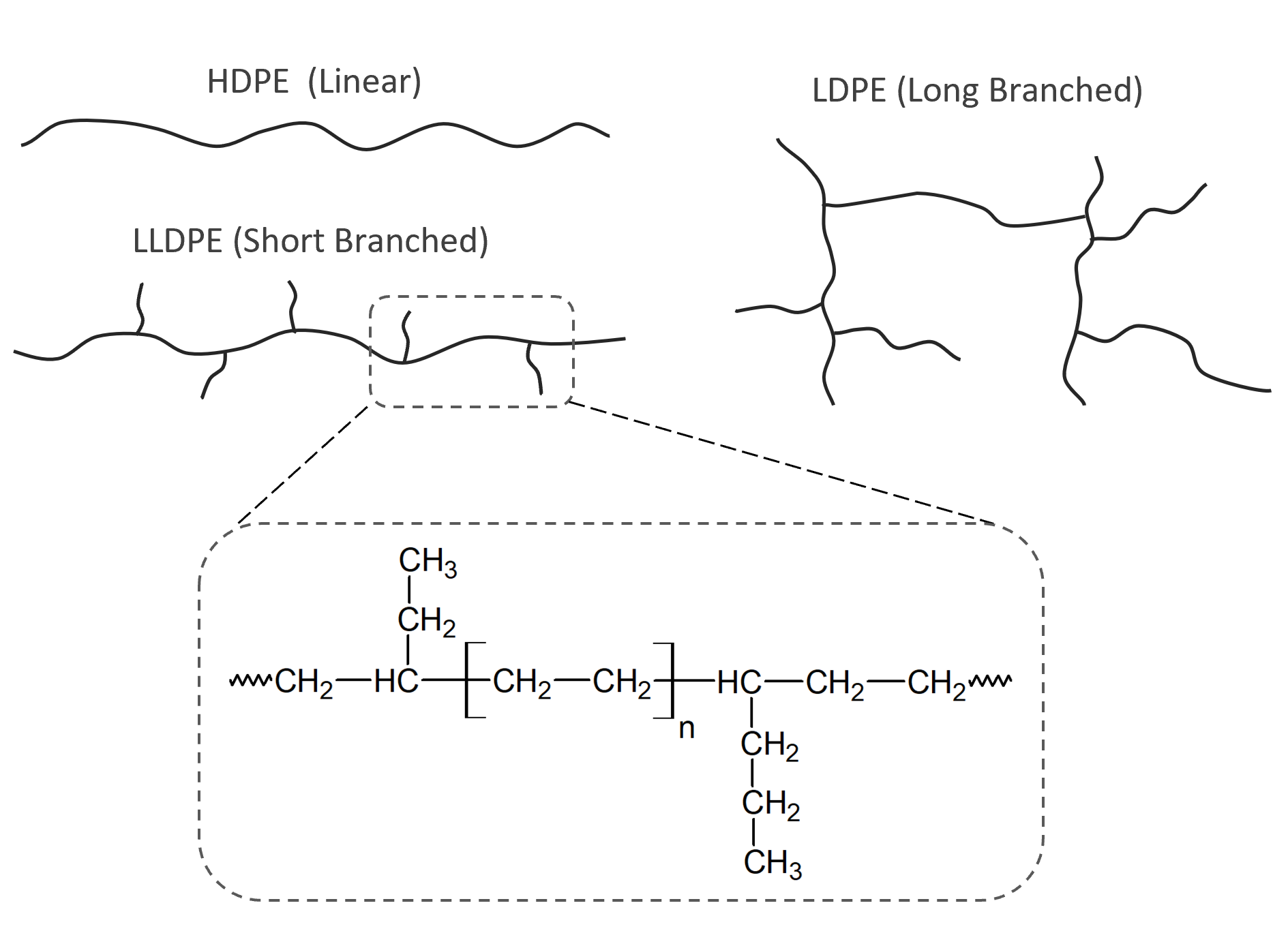
LDPE is a very flexible and tough polyethylene. Compared to HDPE, it has a higher degree of short and long side-chain branching. Branching reduces the tendency of the molecules to pack closely together in hard, stiff, crystalline domains which results in lower density and crystallinity as well as in greater flexibility and toughness. However, LDPE has a significantly lower tensile strength, heat deflection temperature and melting point than HDPE. Compared to LLDPE, it has smaller crystallites due to longer side and subside branching. The degree of crystallinity is usually in the range of 40% to 55% depending on branching and thermal history.
LLDPE has
similar strength as HDPE but is much more flexible. The polymer
chains have a large number of short branches, which are
shorter and more abundant than those of LDPE. This allows the
chains to easily slide against each other under stress without
becoming entangled. LLDPE grades generally have a
narrower molecular weight distribution (polydispersity) than LDPE. Both the absence of long side chains
and the narrower distribution increases the degree of crystallinity which results in higher tensile and impact strength and
greater puncture resistance compared to LDPE. This type of low
density polyethylene
can be processed into thinner films with better
environmental stress crack resistance than HDPE and LDPE.
HDPE has a much lower degree of branching than LDPE which results in a much higher degree of crystallinity typically in the range of 70% to 80% depending on molecular weight and thermal history. The crystals are also larger and more uniform than those of LDPE. HDPE is much denser, more rigid and less permeable than the other types of polyethylene with the exceptions of HMWPE and UHMWPE. It is much stronger and harder than LDPE but it is also less tough and flexible and has lower stress crack resistance. HDPE is mainly used for applications where high strength, rigidity and/or high chemical resistance are required.
VLDPE, ULDPE are very- (0.89 - 0.914 gm/cc) and ultra- (0.889 gm/cc) low density polyethylenes. They have a higher alpha-olefin content than the other types of polyethylene. The flexible alkyl branches of the alpha-olefins lower the packing density, and crystallinity which, in turn, results in high toughness and elasticity but very low tensile strength.
XLPE is a cross-linkable polyethylene. It is a high density, chemically modified polyethylene which has reactive side groups. This allows these grades to be crosslinked by moisture, radiation, catalysts, or with chemicals (Polidan® PEX/XLPE). The crosslinking converts polyolefins into insoluble and infusible polymers which have improved impact strength as well as improved creep, abrasion and stress crack resistance.
Polypropylene (PP) is the
second largest sales volume commodity thermoplastic. The
majority of polypropylenes are isotactic (i-PP). They often have
a level of crystallinity between that of LDPE and HDPE. PP's
elongation at break is similar to that of LDPE, whereas its
impact strength and tensile modulus is closer to that of HDPE.
Despite its semicrystalline nature, it is relatively easy to
mould.
Polypropylene has a lower density than most of the
polyethylenes (0.9 g/cc) and a significantly higher melting
temperature (160 - 180°C) which makes it more suitable for
higher temperature applications such as retortable plastic
products. However, i-PP has lower impact resistance. To reduce
its brittleness, it is sometimes copolymerized with ethylene,
which leads to improved toughness and flexibility.
Syndiotactic polypropylene is produced on a much
smaller scale. It is often less crystalline but has greater
clarity, elasticity, and impact toughness.
Ethene and propene can be copolymerized with higher n-olefins, cyclic olefins or polar monomers. The properties of these copolymers can vary considerably, thus extending the field of possible applications.
Other noteworthy polyolefins are polyisobutylene (PIB) and polybutylene (polybutene-1, PB-1). These polymers are produced on a much smaller scale. The lower molecular weight grades are viscous liquids or sticky solids, and the high molecular grades are rubbery materials. Isobutylene is often copolymerized with a small amount of isoprene. This random copolymer is called butyl rubber (IIR). It is known for its excellent impermeability and good flexibilty. Polybutylene (PB-1) is the polymer of 1-butene. It has very good stability against stress cracking and corrosion and is weldable. It is often blended with other polyolefins to tailor their thermal bonding properties and peel strength.
| Polyolefin | Abreviation | Density - gm/cc |
| Ultra Low Density Polyethylene | ULDPE | 0.867 - 0.889 |
| Very Low Density Polyethylene | VLDPE | 0.890 - 0.914 |
| Low Density Polyethylene | LDPE | 0.919 - 0.925 |
| Linear Low Density Polyethylene | LLDPE | 0.919 - 0.925 |
| Medium Density Polyethylene | MDPE | 0.926 - 0.940 |
| Linear Medium Density Polyethylene | LMDPE | 0.926 - 0.940 |
| High Density Polyethylene | HDPE | 0.941 - 0.970 |
COMMERCIAL Polyolefins
Polyolefins are produced by a large number of companies. Some major producers and trade names are:
Polypropylene:- LyondellBasell Industries: Hostalen® PP, Adstif® PP, Pro-Fax® PP,
- Saudi Basic Industries Corp.: Sabic® PP, SABIC® Verstolen
- Ineos Group Lim.: Ineos PP, Eltex® PP
- ExxonMobil: Exceed™, ExxonMobil™ PP, Exxtral™ PP
- LyondellBasell Industries: , Lupolen® PE, Plexar® PE Alathon® PE and Petrothene® PE
- Saudi Basic Industries Corp.: Sabic® PE, Sabic® HDPE, Sabic® LLDPE
- Ineos Group Lim.: Eltex® PE, Rigidex®, PE, Ineos HDPE
- EXXONMobil: Enable™, ExxonMobil® LLDPE LL, NTX™, Exceed™
- Dow Chemical Company: Flexomer™ , Tuflin™ , DOW™ LDPE , Attane™ , Unival™
Applications
Polyethylene is the most widely used commodity thermoplastic. On a volume basis, HDPE is the most important grade, followed by LLDPE and LDPE. Applications include those in the building and construction, consumer goods, electronics, electrical, and automotive industries. PE is widely used for manufacturing various consumer goods, including containers, buckets, pipes, toys, covers, lids, plastic bags, stretch wraps, plastic wraps, sheets, films, bottles and extrusion coated paper cartons.
The second most important polyolefin is isotactic polypropylene (i-PP). It finds uses in the same industries as PE. i-PP competes in many areas with HDPE. In comparison to HDPE, it has a higher melting point, better crack resistance, a higher heat deflection temperature, and in many cases, higher tensile strength. i-PP has superior stress crack resistance. It is, however, less stable than HDPE to thermal, light, UV and oxidative degradation. i-PP is often the better choice for more demanding applications, unless a higher resistance against thermooxidative degradation is required.
Polyisobutene (PIB) is produced on a much smaller scale. The low molecular grades are viscous oily liquids, the mid-range are sticky products, and the high molecular grades are elastic rubbery materials. PIB is used for adhesives, sealants, chewing gum, roofing membranes, lubricants, cable filling, and synthetic rubber (butyl rubber).
Another low volume polyolefin is polybutene-1 (PB-1). It is highly compatible with polypropylene due to its similar molecular structure and can be used to improve its mechanical properties at elevated temperatures. Other major applications are hot and cold (drinking) water pressure pipes as well as consumer and medical packaging. Low molecular weight grades are used in adhesives, sealants, paper laminates and tapes to improve tack and peel strength. It is (fairly) compatible with many nonpolar resins including PE, PP, EVA, SBS, and SIS.